rpc00001: Nicotiana tabacum BY-2 cell suspension culture
Note
Components
Domestic delivery: A 50-mL plastic conical centrifuge tube, containing cell suspension
Overseas delivery: A 250-mL plastic Erlenmeyer flask, containing cells placed on semi-solid medium
Notice
Subculture the cells to fresh medium immediately after arrival.
Do not store the cell culture in a refrigerator and a freezer.
Maintain aseptic conditions of the cell culture, and work in a laminar flow cabinet.
Method
Culture medium: mLS medium, 0.2 mg/L 2,4-D, pH 5.8 (medium no. 1)
Culture conditions: 27°C, dark, 130 rpm
Subculture: 7-day intervals
Citation of cell line
When results obtained by using this cell line are published in a scientific journal, it should be cited in the following manner: “Nicotiana tabacum BY-2 cell line (rpc00001) was provided by the RIKEN BRC through the National BioResource Project of the MEXT, Japan.”
Introduction
Tobacco BY-2 cell line is a fast-growing and highly-homogeneous cell line, and used for a broad range of plant studies around the world (Nagata et al. 2006 [2]). A suspension culture was established from a callus induced from a seedling of Nicotiana tabacum L. cultivar Bright Yellow 2 (Nagata et al. 1992 [3]). The BY-2 cells are grown in a modified Linsmaier and Skoog (mLS) medium supplemented with 0.2 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), pH 5.8. Our BY-2 cell culture has been maintained in the dark at 27°C with rotary shaking at 130 rpm and subcultured at 7-day intervals (Figure 1, Figure 2).
Materials
Chemicals and stock solutions
(All stock solutions are stored at 4°C)
MS salt mix
Murashige and Skoog Plant Salt Mixture, FUJIFILM Wako Pure Chemical Corporation (#392-00591)
Sucrose
BY2_P
Chemical
Concentration (mg/mL)
KH2PO4
80
LS_VT_modified
Chemical
Concentration (mg/mL)
Thiamine·HCl
0.4
myo-Inositol
40
2,4-D (0.2 mg/mL)
Chemical
Concentration (mg/mL)
2,4-D sodium monohydrate
0.236
(2,4-Dichlorophenoxy)acetic acid sodium salt monohydrate, Sigma-Aldrich (D6679)
KOH (1 N)
Glassware
Erlenmeyer flask (300 mL), capped with two layers of aluminum foil
Pipette (10 mL; large tip opening) and a bulb, sterilized by autoclaving at 121°C for 20 min
Preparation of mLS medium (medium no. 1)
Dissolve the following chemicals in approximately 800 mL of distilled water.
Chemical
Amount
MS salt mix
1 bag (1 L)
Sucrose
30 g
Add following stock solutions, and fill up to approximately 950 mL with distilled water.
Stock solution
Volume (mL)
BY2_P
2.5
LS_VT_modified
2.5
2,4-D (0.2 mg/mL)
1
Adjust the pH of the solution to 5.8 with KOH (1 N), and fill up to 1 L with distilled water.
Pour 95 mL of the medium into a 300-mL flask.
Autoclave the flask at 121°C for 20 min.
Methods
Agitate a 7-day-old culture well and transfer 1–1.2 mL of cell suspension to 95 mL of fresh mLS medium with a pipette.
Incubate cell cultures on a rotary shaker at 130 rpm under the dark condition at 27°C.
Notes
For domestic customers: We send BY-2 cell suspension in a 50-mL disposable conical centrifuge tube. The cells should be transferred to fresh mLS medium immediately after arrival.
For overseas customers: We send BY-2 cells placed on semi-solid mLS medium in a 250-mL disposable Erlenmeyer flask. To re-establish cell suspension cultures, refer to the culture initiation protocol.
In order to maintain BY-2 cell suspension cultures stably, it is essential to transfer an adequate amount of cells to fresh mLS medium in every subculture. The amount of cells may vary from one lab to another, because proliferation of BY-2 cells is affected by culture conditions, such as a room temperature, rotation speed of a rotary shaker, and aeration condition of the culture.
A low growth rate of BY-2 cells is sometimes caused by poor aeration (Kumagai-Sano et al. 2007 [1]). In order to obtain good aeration of a suspension culture, a silicone sponge plug may be used instead of the aluminum foil cap (e.g., cap-type Silicosen; Shin-Etsu Polymer, Tokyo, Japan).
References
Genotyping
Maintenance history
Protocols
External links
Figures
Figure 1

Nicotiana tabacum BY-2 cell suspension culture
Seven-day-old cell suspension culture.
Morphology of BY-2 cells after 3 day of subculture. BY-2 cells were observed by using a differential interference contrast microscope. Scale bar = 100 µm
Figure 2
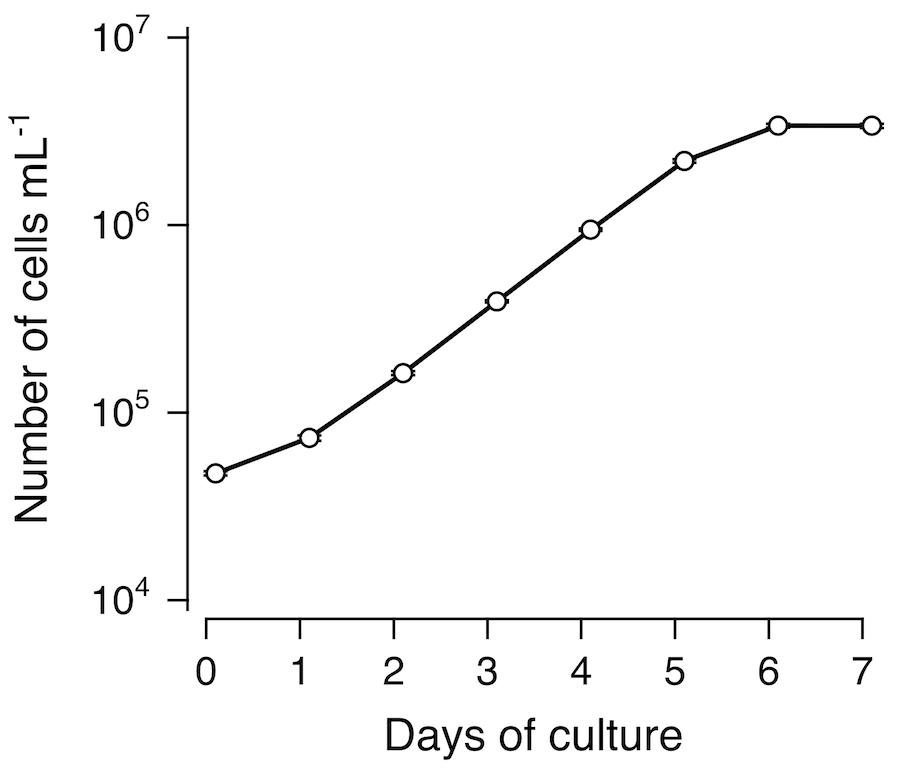
Growth profile of BY-2 cells