Protocol for overseas customers
Re-establishment of suspension cultures of tobacco BY-2 and related cell lines
Note
Cell lines
rpc00039: Nicotiana tabacum GV7 transgenic cell suspension culture
rpc00040: Nicotiana tabacum GF11 transgenic cell suspension culture
rpc00041: Nicotiana tabacum GT16 transgenic cell suspension culture
rpc00062: Nicotiana tabacum BY-TIPG transgenic cell suspension culture
rpc00091: Nicotiana tabacum TBY2-31/ST transgenic cell suspension culture
rpc00092: Nicotiana tabacum TBY2-31/ST(E) transgenic cell suspension culture
rpc00093: Nicotiana tabacum TBY2-41/ST transgenic cell suspension culture
rpc00095: Nicotiana tabacum TBY2-R31 transgenic cell suspension culture
rpc00097: Nicotiana tabacum TBY2-31/41 transgenic cell suspension culture
rpc00109: Nicotiana tabacum BY-HR transgenic cell suspension culture
Culture medium
Methods
Regrowth of BY-2 cells
Recieve an 250-mL Erlenmeyer flask containing BY-2 cells.


Loosen the screw cap slightly to keep good aeration.
Caution
Avoid microbial contamination.
Incubate the BY-2 cells in the dark at 27°C for 1–2 weeks.
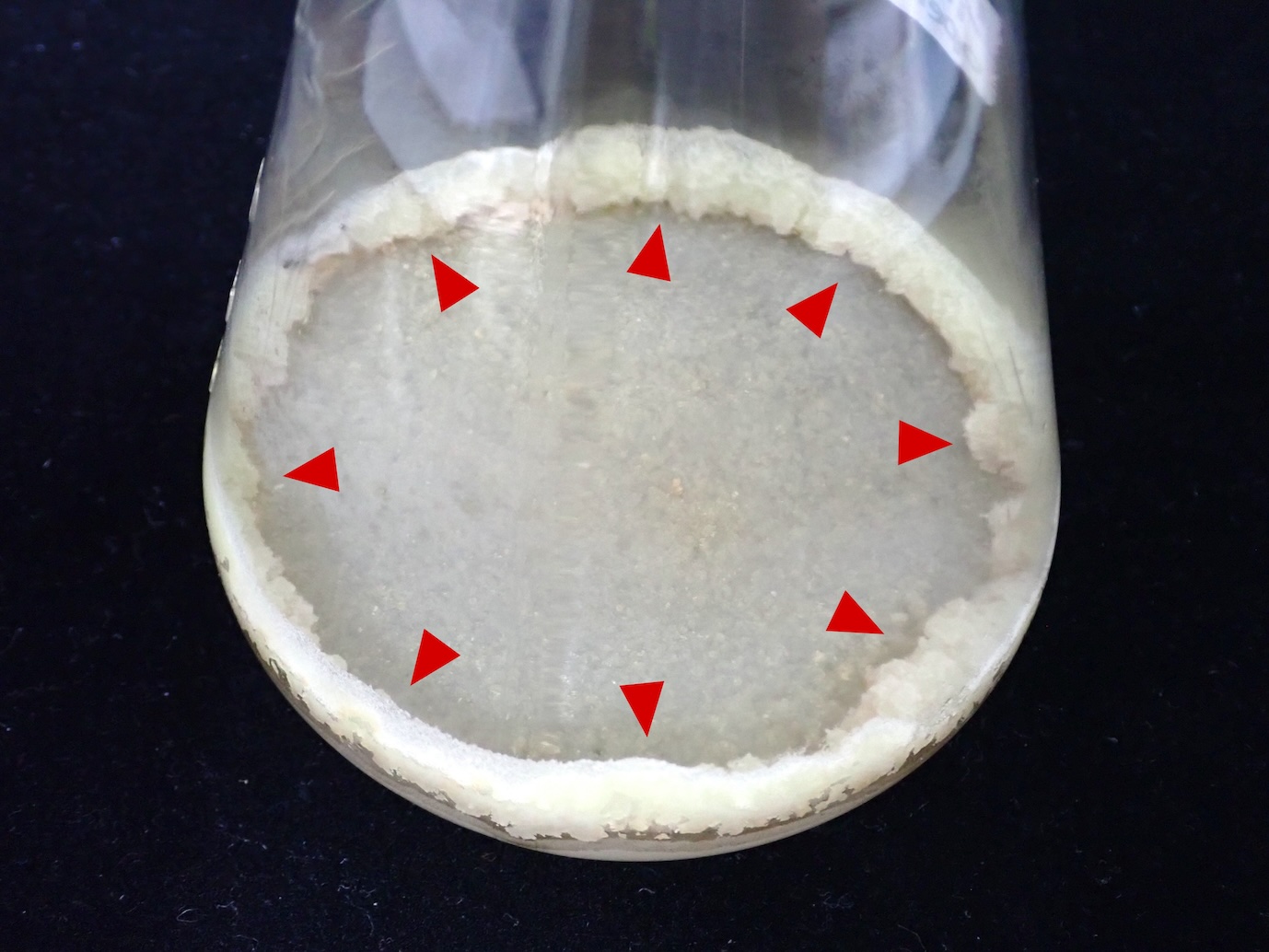
After 8 days. The red arrowheads indicate the growing BY-2 cells.

BY-2 cells around the edge are alive.
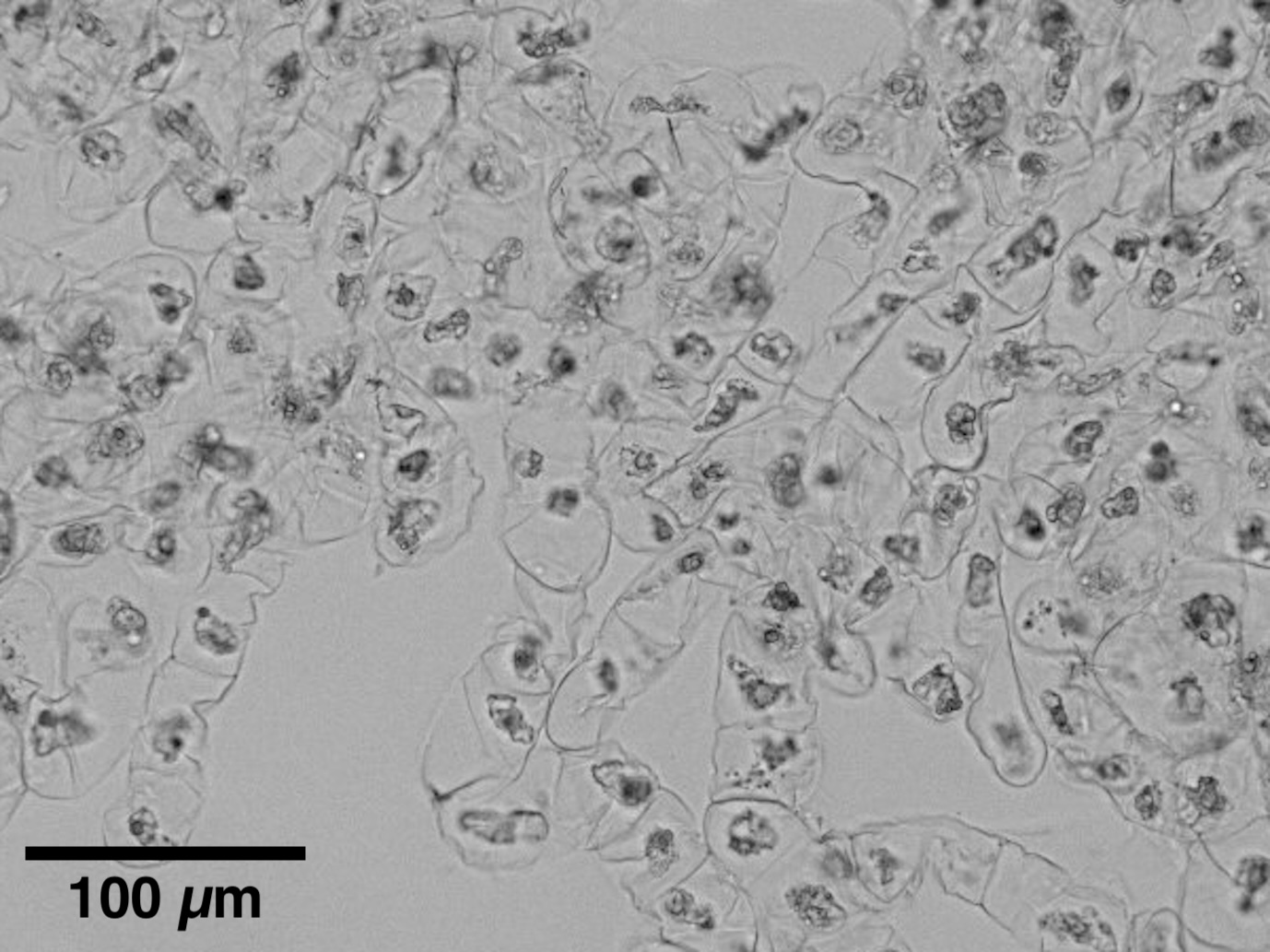
BY-2 cells around the center are dead.
Use the growing BY-2 cells for the induction of suspension cell cultures and for the maintenance of stock agar cultures.
Induction of suspension cell cultures
Transfer the BY-2 cells into a 100-mL Erlenmeyer flask containing 20 mL of a liquid culture medium.
Caution
Use at least the amount of BY-2 cells shown below.

Incubate the BY-2 cells in the dark at 27°C with rotary shaking at 130 rpm for 5–10 days.
Caution
Avoid microbial contamination.
Cover the flask mouth loosely with an aluminum foil cap for good aeration.
Note
A silicone sponge plug can be used instead of an aluminum foil cap (e.g., Cap-type Silicosen; Shin-Etsu Polymer, Tokyo, Japan).

After 7 days
Allow the Erlenmeyer flask to stand for a few minutes just before the first subculture of suspension cell culture.
Transfer 1–2 mL of the sedimented cells into 95 mL of a fresh culture medium in a 300-mL Erlenmeyer flask.
Note
See Appendix A for the BY-2-related cell lines.

Culture the BY-2 cells by the usual method.

After 7 days
After 3–4 subculturing, the BY-2 suspension cell cultures grow stably.
Maintenance of stock agar cultures
Prepare cell culture dishes containing 30 mL of a culture medium solidified with 0.8% (w/v) agar.
Note
BY-2 cells can also be cultured on the media solidified with gellan gum.
Transfer small pieces (3–5 mm in diameter) of BY-2 cells onto agar culture medium.
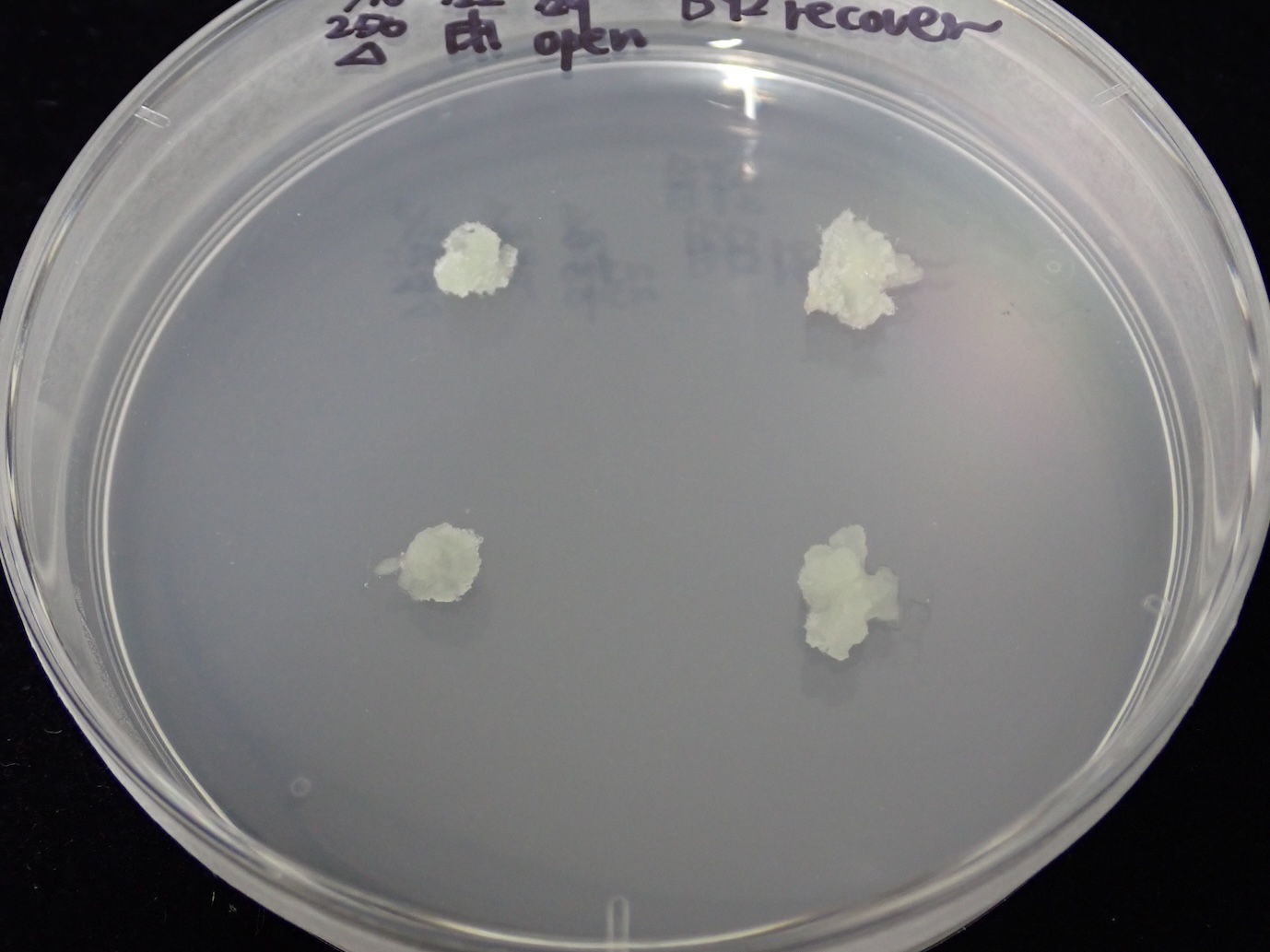
Seal culture dishes by using surgical tape to keep good aeration.
Incubate the BY-2 cells in the dark at 27°C for 2–4 weeks.
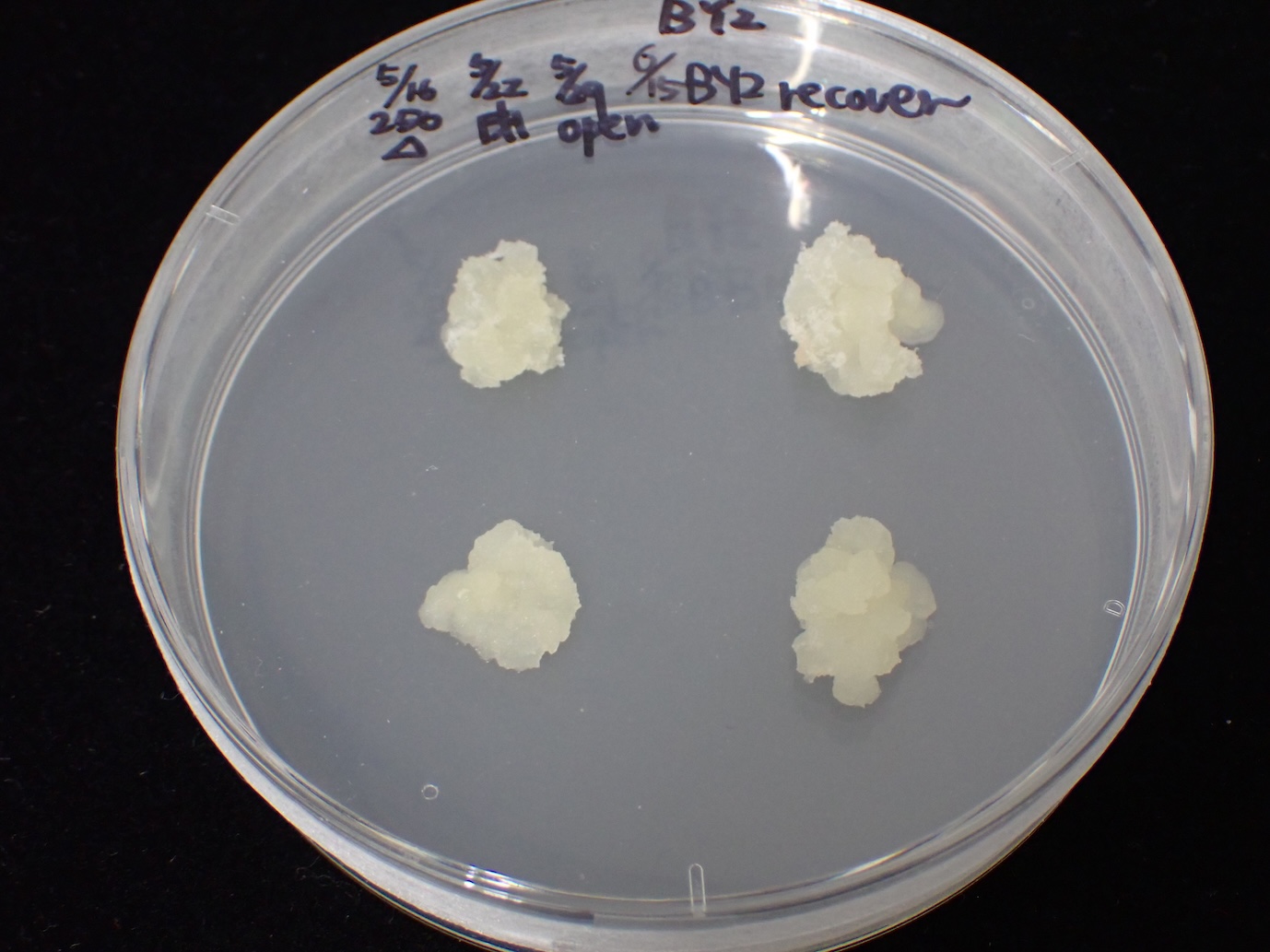
After 4 weeks
Subculture the BY-2 cells on fresh agar medium at 2–4-week intervals.
Appendix A: The first subculture of suspension cell cultures of BY-2-related cell lines
BRC No.
Cell line
Transfer volume (mL) *
Culture medium (mL)
Erlenmeyer flask (mL)
rpc00001
BY-2
1–2
95
300
rpc00039
GV7
1–3
95
300
rpc00040
GF11
3–6
95
300
rpc00041
GT16
3–6
95
300
rpc00062
BY-TIPG
1–3
95
300
rpc00091
TBY2-31/ST
0.4–0.8
30
100
rpc00092
TBY2-31/ST(E)
0.4–0.8
30
100
rpc00093
TBY2-41/ST
0.4–0.8
30
100
rpc00095
TBY2-R31
0.4–0.8
30
100
rpc00097
TBY2-31/41
0.4–0.8
30
100
rpc00109
BY-HR
2–4
95
300
* Subculture sedimented cells to a fresh culture medium.
Caution
For the transgenic BY-2 cell lines, check the expression of fluorescent proteins in re-established suspension cells before use.
Appendix B: Preparation of BY-2 cell cultures for transportation
Preparation of a cell culture
An agar-solidified culture medium was prepared in a 250-mL disposable Erlenmeyer flask.
Note
mLS medium (medium no. 1) solidified with 1.4% (w/v) agar, 80 mL
BY-2 cell suspension culture was prepared on day 7 of the culture.
BY-2 cells were spread on the agar culture medium in the 250-mL disposable Erlenmeyer flask.
The screw cap was loosely closed to keep good aeration.
The BY-2 cells were pre-cultured in the dark at 27°C for 6 days.
The screw cap was tightly closed and sealed with thermoplastic sealing film.
Transportation test
The BY-2 cells was stored in the dark at 27°C for 7 days (simulating transport).
The BY-2 cells were tested for regrowth.