Cryopreservation of tobacco BY-2 suspension cell cultures [1]
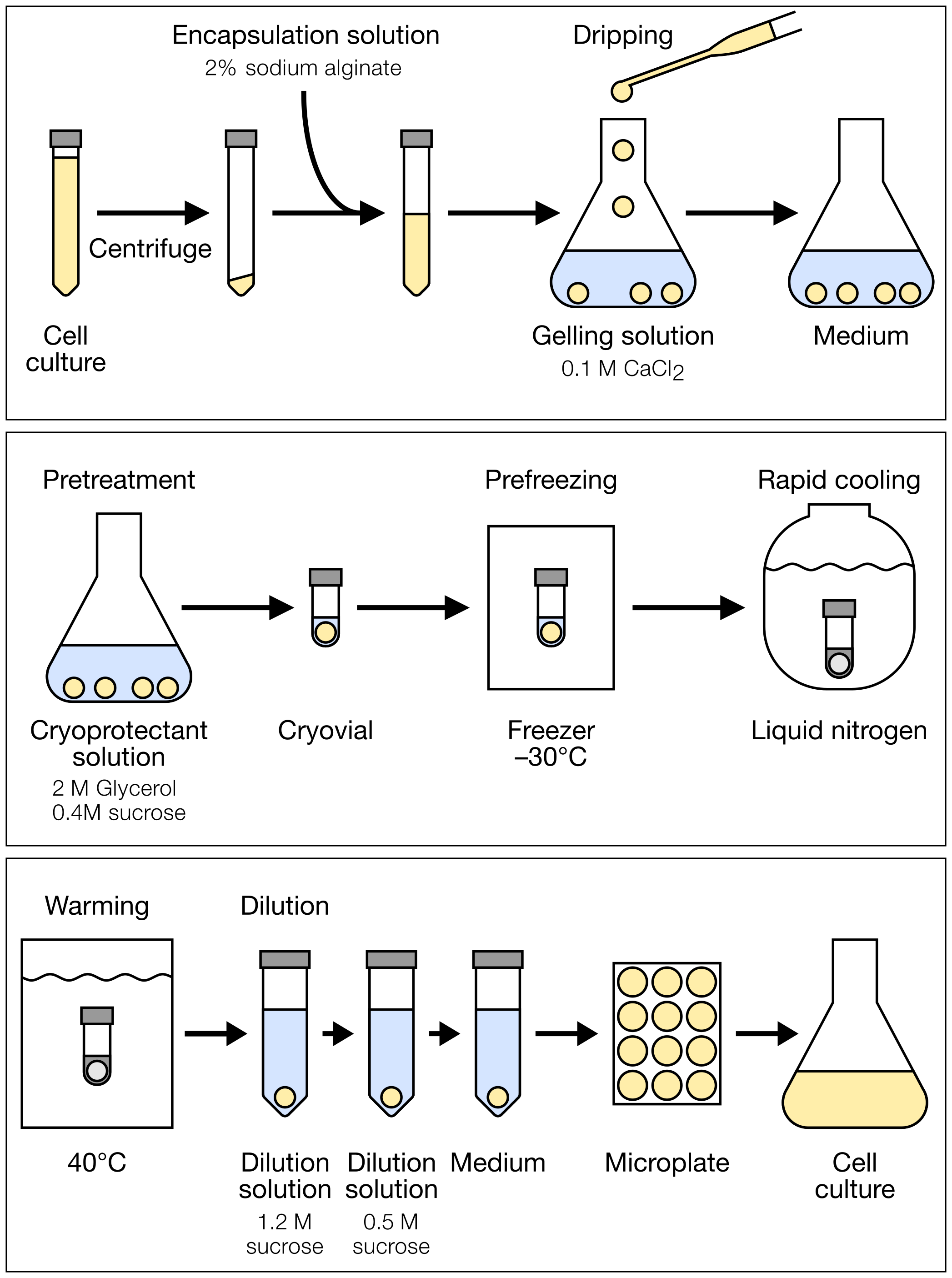
Figure 1: Schematic diagram of cryopreservation procedure
Materials
Plant cell culture
rpc00001: Nicotiana tabacum BY-2 cell suspension culture, after 3 days of subculturing [2]
Chemicals
Cryopreservation
Culture medium: modified Linsmaier and Skoog (mLS) medium, 0.2 mg/L 2,4-dichlorophenoxyacetic acid, pH 5.8 (medium no. 1)
Chemical/Stock solution
For 1 L
MS Plant Salt Mixture
1 bag
Sucrose
30 g
BY2_P
2.5 mL
LS_VT_modified
2.5 mL
2,4-D (0.2 mg/mL)
1 mL
H2O
Adjust pH to 5.8, sterilize by autoclave.
Encapsulation solution: medium containing 2% (w/v) sodium alginate
3 M CaCl2 solution
Chemical/Stock solution
For 50 mL
CaCl2·H2O
22.1 g
H2O
Sterilize by filtration or autoclave.
Gelling solution: medium containing 0.1 M CaCl2
Stock solution
Culture medium, sterilized
60 mL
3 M CaCl2 solution, sterilized
2 mL
2× Medium: double-strength mLS medium, not containing sucrose
Chemical/Stock solution
For 500 mL
Murashige and Skoog Salt Mixture
1 bag
BY2_P
2.5 mL
LS_VT_modified
2.5 mL
2,4-D (0.2 mg/mL)
1 mL
H2O
Cryoprotectant solution: medium containing 2 M glycerol and 0.4 M sucrose
Chemical/Stock solution
For 300 mL
2× Medium
150 mL
Glycerol
55.3 g
Sucrose
41.1 g
H2O
Adjust pH to 5.8, sterilize by autoclave.
Regrowth
Dilution solution (1.2 M): medium containing 1.2 M sucrose
Chemical/Stock solution
For 300 mL
2× Medium
150 mL
Sucrose
123.2 g
H2O
Adjust pH to 5.8, sterilize by autoclave.
Dilution solution (0.5 M): medium containing 0.5 M sucrose
Chemical/Stock solution
For 300 mL
2× Medium
150 mL
Sucrose
51.3 g
H2O
Adjust pH to 5.8, sterilize by autoclave.
Evaluation of cell viability
10 mg/mL Evans blue solution
Chemical/Stock solution
For 10 mL
Evans blue
100 mg
H2O
Staining solution: medium containing 1 mg/mL Evans blue
Stock solution
Culture medium
9 mL
10 mg/mL Evans blue solution
1 mL
Equipment
Cryopreservation
Microscope
Conical tube, 15 mL
Low-speed centrifuge
Pipette
Erlenmeyer flask, 200 mL
Pasteur pipette
Shaker
Cryovial, 2.0 mL, round bottom [5]
Forceps
Vial rack [6]

Figure 2: 1.5 (2) mL tube rack TR-4002 (Micro tube mixer MT-400 supplied rack; TOMY Digital Biology Co., Ltd.)
Laboratory freezer, −30°C
Cane for cryovials
Dewar flask
Regrowth
Conical tube, 50 mL
Water bath
Shaker
Pipette
Forceps
Cell culture plate, 12 well [7]
Micro spatula
Evaluation of cell viability
Surgical blade
Pipette
Cell culture plate, 12 well
Forceps
Microscope slide
Cover slip
Microscope
Methods
Cryopreservation
Check physiological condition of cultured cells by observing them under a microscope. [8]
Transfer suspension cell culture into a 15-mL conical tube.
Centrifuge the tube at 100 ×g for 5 min.
Check volume of the pelleted cells and remove the supernatant with a pipette.
Gently suspend the pelleted cells in 3–4 volume of encapsulation solution.
Pour 60 mL of gelling solution to a 200-mL Erlenmeyer flask.
Drip the mixture of cells and encapsulation solution into the gelling solution with a Pasteur pipette. [9] [10]
Keep the beads formed from the encapsulated cells in the gelling solution for 5–10 min with gentle shaking.
Remove the gelling solution with a pipette.
Wash the beads with 10 mL of culture medium: Add culture medium, gently swirl the Erlenmeyer flask, and remove the culture medium with a pipette.
Incubate the beads in 50 mL of culture medium for 10–20 min.
Remove the culture medium and wash the beads with 10 mL of cryoprotectant solution.
Incubate the beads in 50 mL [11] of cryoprotectant solution at room temperature for 60 min with gentle shaking (pretreatment). [12]
Pour 300 µL of the cryoprotectant solution to a 2-mL cryovial.
Transfer three beads into each cryovial with forceps. [13]
Place the cryovials in a rack and store them in a laboratory freezer at −30°C for 2 h (slow prefreezing). [14] [15]
After removing the cryovials from the freezer, immediately set the cryovials to cryovial canes and immerse it in liquid nitrogen (rapid cooling). [16]
Store the cryovials in vapor phase of a liquid nitrogen storage tank. [17]
Regrowth
Pour 30 mL of dilution solution (1.2 M) to a 50-mL conical tube.
Warm each cryovial in a water bath at 40°C with gentle agitation. [18]
After thawing, immediately remove the cryovials from the bath.
Transfer the three beads and cryoprotectant solution in the conical tube containing dilution solution (1.2 M). [19]
Set the conical tube horizontally on a shaker and incubate the beads at room temperature for 15 min with gentle shaking.
Replace the dilution solution (1.2 M) with 30 mL of dilution solution (0.5 M): Remove the dilution solution (1.2 M) with a pipette and add dilution solution (0.5 M) to the conical tube.
Incubate the beads for 15 min with gentle shaking.
Replace the dilution solution (0.5 M) with 30 mL of culture medium and incubate the beads for 15 min with gentle shaking.
Suspend three beads in 3 mL of fresh culture medium in each well of a 12-well cell culture plate.
Culture the beads at 27°C in the dark for 3 days with shaking at 130 rpm.
Gently crush the beads with a micro spatula to release the encapsulated cells into the culture medium. [20]
Culture the cell suspension for an additional 4 days.
Transfer the cell suspension to 95 mL of fresh culture medium in a 300-mL Erlenmeyer flask.
Evaluation of cell viability
Cut the bead into two to four pieces. [21]
Soak the pieces in 1 mL of Evans blue staining solution in each well of a 12-well cell culture plate for 20 min.
Transfer the pieces to 1 mL of culture medium and incubate them for 20 min.
Place one piece of the bead on a microscope slide and gently crush with a cover slip.
Count living and dead cells under a microscope. [22]